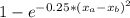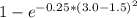Chemistry, 28.01.2020 20:48 Tabbicat021
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding. the electronegativities for and are 1.5 and 3.0 respectively. calculate the fraction of the bonding that is ionic. (enter your answer to three significant figures.) =

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding...
Questions

English, 23.08.2019 07:30


English, 23.08.2019 07:30





Mathematics, 23.08.2019 07:30

Chemistry, 23.08.2019 07:30

Mathematics, 23.08.2019 07:30

History, 23.08.2019 07:30

English, 23.08.2019 07:30






Social Studies, 23.08.2019 07:30