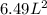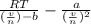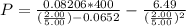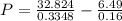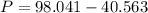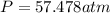Chemistry, 28.01.2020 23:45 marinahuntg
What would be the pressure of a 5.00 mol sample of cl₂ at 400.0 k in a 2.00 l container found using the van der waals equation? for cl₂, a = 6.49 l²・atm/mol² and b = 0.0652 l/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

You know the right answer?
What would be the pressure of a 5.00 mol sample of cl₂ at 400.0 k in a 2.00 l container found using...
Questions



Geography, 21.02.2021 04:30



Mathematics, 21.02.2021 04:30

Mathematics, 21.02.2021 04:30




Business, 21.02.2021 04:30

Mathematics, 21.02.2021 04:30

Mathematics, 21.02.2021 04:30



History, 21.02.2021 04:30