
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻¹s⁻¹ : > 2so₃g + 2so₂g o₂g suppose a vessel contains so₃ at a concentration of 1.44m . calculate the concentration of so₃ in the vessel 0.240 seconds later. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻...
Questions

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00


History, 18.12.2020 14:00

English, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

History, 18.12.2020 14:00



Engineering, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00




Mathematics, 18.12.2020 14:00

Biology, 18.12.2020 14:00

Physics, 18.12.2020 14:00

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/1273/ccade.png)
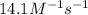
![[A_t]](/tpl/images/0479/1273/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/1273/dc622.png) = initial concentration = 1.44 M
= initial concentration = 1.44 M![14.1\times 0.240=\frac{1}{[A_t]}-\frac{1}{1.44}](/tpl/images/0479/1273/20832.png)
![[A_]t=0.24M](/tpl/images/0479/1273/ae6b5.png)


