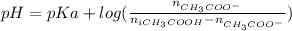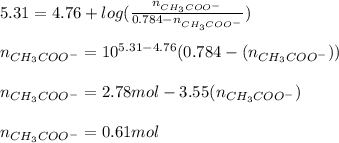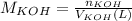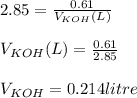Chemistry, 29.01.2020 01:45 jacksonyodell8601
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution and a 2.85 m koh 2.85 m koh solution. if you have 930 ml 930 ml of the acetic acid solution, how many milliliters of the koh koh solution do you need to add to make a buffer of ph 5.31 5.31 ? the p k a pka of acetic acid is 4.76. 4.76.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution an...
Questions






Computers and Technology, 13.11.2019 17:31














![pH=pKa + log(\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]})](/tpl/images/0479/5908/7f7e8.png)