Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temp...

Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temperature it obeys this rate law.
rate = (6.48 m-1 • s-1)[cl2o5]2
suppose a vessel contains cl2o5 at a concentration of 1.16 m. calculate the concentration of cl2o5 in the vessel 0.820 seconds later. you may assume no other reaction is important.
round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
Questions

Mathematics, 25.02.2021 01:00


English, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00






Mathematics, 25.02.2021 01:00

Biology, 25.02.2021 01:00

Computers and Technology, 25.02.2021 01:00

Arts, 25.02.2021 01:00






Mathematics, 25.02.2021 01:00

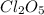 in the vessel 0.820 seconds later is, 0.16 M
in the vessel 0.820 seconds later is, 0.16 M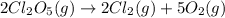
![rate=(6.48M^{-1}s^{-1})[Cl_2O_5]^2](/tpl/images/0479/5701/ef798.png)
![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/5701/ccade.png)
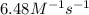
![[A_t]](/tpl/images/0479/5701/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/5701/dc622.png) = initial concentration = 1.16 M
= initial concentration = 1.16 M![6.48\times 0.820=\frac{1}{[A_t]}-\frac{1}{1.16}](/tpl/images/0479/5701/3da76.png)
![[A_t]=0.16M](/tpl/images/0479/5701/3ee9b.png)


