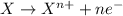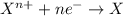Chemistry, 29.01.2020 02:52 katelynamber10
Acalled a reductant or reducer) is an element or compound that loses (or "donates") an electron to an electron recipient (oxidizing agent) in a redox chemical reaction.
a reducing agent is thus oxidized when it loses electrons in the redox reaction. reducing agents "reduce" (or, are "oxidized" by) oxidizing agents. oxidizers "oxidize" (that is, are reduced by) reducers.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Acalled a reductant or reducer) is an element or compound that loses (or "donates") an electron to a...
Questions

Mathematics, 23.05.2020 00:04



Mathematics, 23.05.2020 00:04


History, 23.05.2020 00:05

English, 23.05.2020 00:05






Mathematics, 23.05.2020 00:05

Mathematics, 23.05.2020 00:05

Mathematics, 23.05.2020 00:05

Biology, 23.05.2020 00:05




Chemistry, 23.05.2020 00:05