
Chemistry, 29.01.2020 02:45 gizmo50245
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated temperature to yield 0.692 g h₂o and 3.381 g co₂. (a) calculate the masses of c and h in the sample. (b) does the compound contain any other elements

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated tempe...
Questions


Geography, 10.03.2021 04:00

History, 10.03.2021 04:00



Chemistry, 10.03.2021 04:00



Mathematics, 10.03.2021 04:00

Chemistry, 10.03.2021 04:00


English, 10.03.2021 04:00


Mathematics, 10.03.2021 04:00

Social Studies, 10.03.2021 04:00


Mathematics, 10.03.2021 04:00

Mathematics, 10.03.2021 04:00

Chemistry, 10.03.2021 04:00

Mathematics, 10.03.2021 04:00

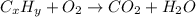
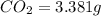
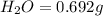
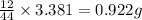 of carbon will be contained.
of carbon will be contained.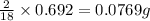 of hydrogen will be contained.
of hydrogen will be contained.

