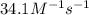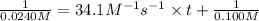Chemistry, 29.01.2020 03:52 njohns5327home
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of 34.1 m^-1*s^-1:
nh4oh(aq) > nh3(aq) + h2o (aq)
suppose a vessel contains nh4oh at a concentration of 0.100m. calculate how long it takes for the concentration of nh4oh to decrease to 0.0240 m. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1


Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of...
Questions





Social Studies, 01.07.2021 16:50









Mathematics, 01.07.2021 16:50


Computers and Technology, 01.07.2021 16:50




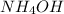 to decrease to 0.0240 M.
to decrease to 0.0240 M.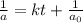
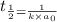
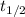 = half life of reaction
= half life of reaction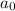 = initial concentration
= initial concentration 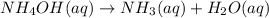
![[NH_4OH]=a_o=0.100 M](/tpl/images/0480/1114/fa6e4.png)
![[NH_4OH]=a=0.0240 M](/tpl/images/0480/1114/c0e10.png)