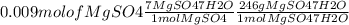Chemistry, 29.01.2020 04:50 taylabrown2013
On the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked to make 30 ml of 0.3 m mgso4. the formula on the bottle is mgso4∗7h2o (also known as epsom salt). calculate the amount of salt you need (in milligrams).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
On the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked...
Questions





Biology, 28.09.2019 04:30



Biology, 28.09.2019 04:30





Mathematics, 28.09.2019 04:30

Computers and Technology, 28.09.2019 04:30

Mathematics, 28.09.2019 04:30

History, 28.09.2019 04:30


Biology, 28.09.2019 04:30

History, 28.09.2019 04:30