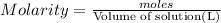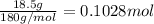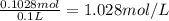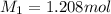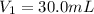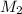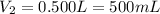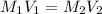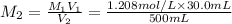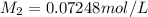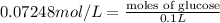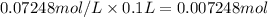Chemistry, 29.01.2020 04:47 kenzierosa
Astudent placed 18.5 g of glucose (c6h12o6) in a volumetric flask, added enough water to dissolve the glucose by swirling, then carefully added additional water until the 100. ml mark on the neck of the flask was reached. the flask was then shaken until the solution was uniform. a 30.0 ml sample of this glucose solution was diluted to 0.500 l. how many grams of glucose are in 100. ml of the final solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Astudent placed 18.5 g of glucose (c6h12o6) in a volumetric flask, added enough water to dissolve th...
Questions

Mathematics, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01



Health, 28.07.2020 21:01


Mathematics, 28.07.2020 21:01

History, 28.07.2020 21:01

History, 28.07.2020 21:01

English, 28.07.2020 21:01





Business, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

Social Studies, 28.07.2020 21:01

Physics, 28.07.2020 21:01

History, 28.07.2020 21:01