
Chemistry, 29.01.2020 05:40 herringalyssa
What volume of hydrogen gas is required to react with 113 liters of ethylene (c2h4) according to the following reaction? (all gases are at the same temperature and pressure.) hydrogen (g) + ethylene (c2h4) (g) ethane (c2h6) (g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
What volume of hydrogen gas is required to react with 113 liters of ethylene (c2h4) according to the...
Questions

Biology, 20.01.2020 11:31

Spanish, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

Computers and Technology, 20.01.2020 11:31


History, 20.01.2020 11:31




Mathematics, 20.01.2020 11:31

Biology, 20.01.2020 11:31

Mathematics, 20.01.2020 11:31

English, 20.01.2020 11:31


Social Studies, 20.01.2020 11:31


Mathematics, 20.01.2020 11:31

English, 20.01.2020 11:31

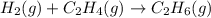
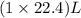 of ethylene reacts with
of ethylene reacts with 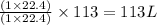 of hydrogen gas
of hydrogen gas

