
Chemistry, 29.01.2020 05:44 zodiacpumpkin1126
It takes 24.56 ml of a sodium hydroxide solution to precisely neutralize 2.023 g of potassium hydrogen phthalate (f. w. = 204.22g/mol). what is the concentration of the sodium hydroxide solution?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
It takes 24.56 ml of a sodium hydroxide solution to precisely neutralize 2.023 g of potassium hydrog...
Questions





Biology, 05.06.2020 02:02


Mathematics, 05.06.2020 02:02

Biology, 05.06.2020 02:02



Mathematics, 05.06.2020 02:02




Mathematics, 05.06.2020 02:02





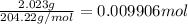
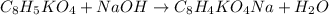
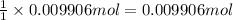 of NaOH
of NaOH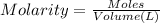
![[NaOH]=\frac{0.009906 mol}{0.02456 L}=0.4033 M](/tpl/images/0480/5312/e10a7.png)



