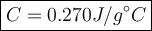Chemistry, 29.01.2020 14:40 alonnachambon
2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0°c. when the system came to thermal equilibrium, the temperature
was 15.3°c. what is the specific heat capacity of molybdenum? the specific heat
capacity of water is 4.184 j/g. k. (2.5 points)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0...
water at 10.0...
Questions








Social Studies, 26.02.2020 00:33




Computers and Technology, 26.02.2020 00:33

Mathematics, 26.02.2020 00:33

Chemistry, 26.02.2020 00:33