how many moles of hydrogen are needed to produce 13.78 mol of ethane?

Chemistry, 22.09.2019 13:30 versaceblooper
C2h2+2h2 yields c2h6
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
pls

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1
You know the right answer?
C2h2+2h2 yields c2h6
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
Questions

Chemistry, 07.04.2021 07:40


Mathematics, 07.04.2021 07:40

English, 07.04.2021 07:40

Mathematics, 07.04.2021 07:40


Mathematics, 07.04.2021 07:40




Advanced Placement (AP), 07.04.2021 07:40


Mathematics, 07.04.2021 07:40

Arts, 07.04.2021 07:40

Mathematics, 07.04.2021 07:40



Mathematics, 07.04.2021 07:40


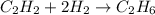
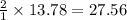 moles of hydrogen
moles of hydrogen 


