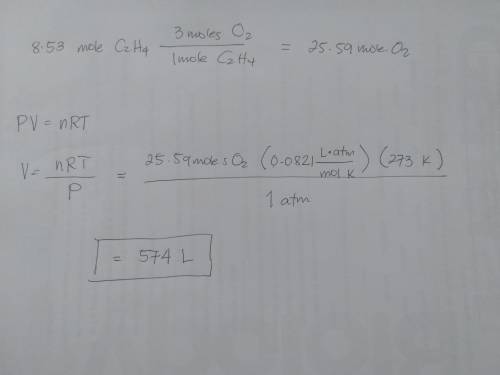
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with...

Chemistry, 02.02.2020 12:42 DonovanBaily42
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with o2, according to the following equation:
c2h4(g) + 3o2(g) → 2co2(g) + 2h2o(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Questions

Mathematics, 21.07.2019 21:20


Mathematics, 21.07.2019 21:20



Chemistry, 21.07.2019 21:20


Mathematics, 21.07.2019 21:20

Health, 21.07.2019 21:20

History, 21.07.2019 21:20






Chemistry, 21.07.2019 21:20