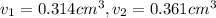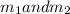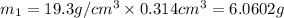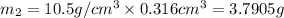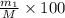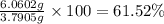Chemistry, 11.11.2019 06:31 kimloveswim
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, repectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains. calculate the percentage of gold(by mass) in the jewelry. (b) the relative amount of gold in an alloy is commonly expressed in units of karats.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a...
Questions

Arts, 21.01.2021 22:00


English, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00





Mathematics, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00

Physics, 21.01.2021 22:00



History, 21.01.2021 22:00


Spanish, 21.01.2021 22:00

Biology, 21.01.2021 22:00

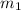
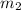
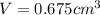
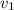
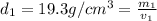
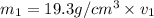
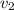
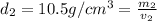
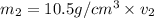
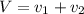
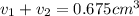 ..(1)
..(1)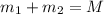
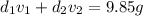
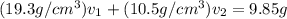 ..(2)
..(2)