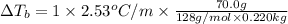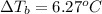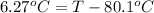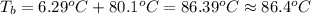Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a nonvolatile nonelectrolyte), in 220.0 g of benzene, c6h6. the kb for benzene = 2.53oc/m. the boiling point of pure benzene is 80.1oc.. ans: 86.4 degrees celsius. i did this so far. 1)70g c10h8(1mol c10h8/128gc10h8)= .546mol c10h8. benzene)=2.482m. 3) (2.482)(2.53 c/m)=6.289. i'm not sure if i am starting this off right, can anyone me get the correct answer? ans ty!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a non...
Questions




Mathematics, 14.08.2020 23:01


Mathematics, 14.08.2020 23:01

Physics, 14.08.2020 23:01




Mathematics, 14.08.2020 23:01



History, 14.08.2020 23:01

Computers and Technology, 14.08.2020 23:01


Mathematics, 14.08.2020 23:01



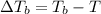
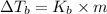
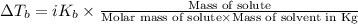
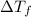 =Elevation in boiling point
=Elevation in boiling point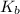 = Boiling point constant of solvent = 2.53 °C/m(benzene)
= Boiling point constant of solvent = 2.53 °C/m(benzene)