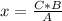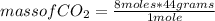Chemistry, 21.08.2019 18:40 serenityarts123
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane reacts with excess oxygen.. express your answer to three significant figures and include the appropriate units.. calculate the mass of butane needed to produce 72.7g of carbon dioxide.. express your answer to three significant figures and include the appropriate units.. how do you do it?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane react...
Questions

History, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20


Mathematics, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20




Physics, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20


Computers and Technology, 22.01.2021 19:20

Social Studies, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20



Mathematics, 22.01.2021 19:20

Mathematics, 22.01.2021 19:20