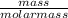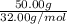Passing an electric current through a sample of water (h2o) can cause the water to decompose into hydrogen gas (h2) and oxygen gas (o2) according to the following equation.
2h2o mc026-1.jpg 2h2 + o2
the molar mass of h2o is 18.01 g/mol. the molar mass of o2 is 32.00 g/mol. what mass of h2o, in grams, must react to produce 50.00 g of o2?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
Passing an electric current through a sample of water (h2o) can cause the water to decompose into hy...
Questions


Business, 02.07.2019 15:30


Mathematics, 02.07.2019 15:30


Mathematics, 02.07.2019 15:30



Mathematics, 02.07.2019 15:30





Mathematics, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

Physics, 02.07.2019 15:30

Social Studies, 02.07.2019 15:30