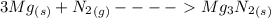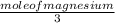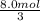Chemistry, 19.11.2019 18:31 whitneyt3218
The following is a limiting reactant problem:
magnesium nitride is formed in the reaction of magnesium metal with nitrogen gas in this reaction: 3 mg(s) + n2(g) > mg3n2(s)
how many grams of product are formed from 2.0 mol of n2 (g) and 8.0 mol of mg(s)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The following is a limiting reactant problem:
magnesium nitride is formed in the reaction of...
magnesium nitride is formed in the reaction of...
Questions

Mathematics, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00


Mathematics, 24.08.2021 01:00

Mathematics, 24.08.2021 01:00

Advanced Placement (AP), 24.08.2021 01:00





Social Studies, 24.08.2021 01:00








English, 24.08.2021 01:00