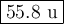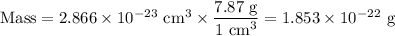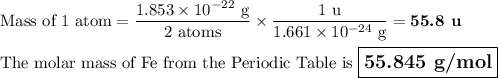Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell whose edge length is 286.6 pm. the density of iron is 7.87 g cm–3 . what is the mass of an iron atom? compare this value with the value you obtain from the molar mass

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell who...
Questions



English, 27.09.2021 03:10


Mathematics, 27.09.2021 03:10

Mathematics, 27.09.2021 03:10


English, 27.09.2021 03:10

Mathematics, 27.09.2021 03:10


Mathematics, 27.09.2021 03:10


Mathematics, 27.09.2021 03:10



Mathematics, 27.09.2021 03:10

Mathematics, 27.09.2021 03:10



English, 27.09.2021 03:10