
Chemistry, 06.02.2020 02:44 ddddre4460
An unknown compound contains only c , h , and o . combustion of 9.30 g of this compound produced 22.7 g co 2 and 9.29 g h 2 o . what is the empirical formula of the unknown compound? insert subscripts as needed. empirical formula: ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
An unknown compound contains only c , h , and o . combustion of 9.30 g of this compound produced 22....
Questions


Mathematics, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40

English, 18.03.2021 01:40


History, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40








Mathematics, 18.03.2021 01:40

Chemistry, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

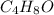
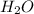 = 9.29 g /18 g/mol = 0.51611 moles
= 9.29 g /18 g/mol = 0.51611 moles
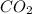 = 22.7 g /44.01 g/mol = 0.5158 moles
= 22.7 g /44.01 g/mol = 0.5158 moles


