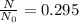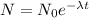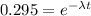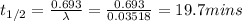Chemistry, 10.02.2020 20:25 kelsotay623
What is the half-life of bismuth-214 if 34.7 minutes are required for the mass of a sample of bismuth-214 to fall to 29.5 percent of its original value? Since the decomposition is a radioactive decay reaction, it is first order.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
What is the half-life of bismuth-214 if 34.7 minutes are required for the mass of a sample of bismut...
Questions



Physics, 03.08.2019 04:30

Mathematics, 03.08.2019 04:30







Social Studies, 03.08.2019 04:30

English, 03.08.2019 04:30

Mathematics, 03.08.2019 04:30





Computers and Technology, 03.08.2019 04:30