Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
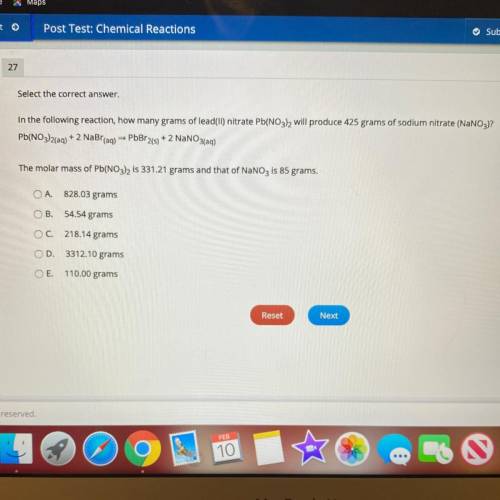
In the following reaction, how many grams of lead(II) nitrate Pb(NO3), will produce 425 grams of sod...
Questions


History, 08.12.2019 16:31


Health, 08.12.2019 16:31

Mathematics, 08.12.2019 16:31

Chemistry, 08.12.2019 16:31



Mathematics, 08.12.2019 16:31


Biology, 08.12.2019 16:31

Biology, 08.12.2019 16:31


Social Studies, 08.12.2019 16:31

Physics, 08.12.2019 16:31

History, 08.12.2019 16:31

History, 08.12.2019 16:31



Biology, 08.12.2019 16:31