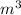A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K...

Chemistry, 10.02.2020 21:07 cordobamariana07
A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K. What is the approximate volume of the balloon? (The
ideal gas law and gas constant are given below.)
PV = nRT
R=8.314 m. Pa/
mol K


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Questions


Chemistry, 14.12.2020 16:40










Business, 14.12.2020 16:40

Chemistry, 14.12.2020 16:40