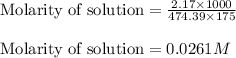Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1



Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
What is the molarity of SO4^2- in a solution prepared by mixing 2.17 g of alum, KAl(SO4)2•12H2O, wit...
Questions



Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



History, 11.10.2020 14:01



Advanced Placement (AP), 11.10.2020 14:01

Advanced Placement (AP), 11.10.2020 14:01

Mathematics, 11.10.2020 14:01







Mathematics, 11.10.2020 14:01