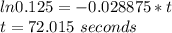The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-life for the reaction at 550°C is 24 seconds. How many seconds does it take for the formic acid concentration to decrease by 87.5%? (1) 4.6 seconds (2) 36 seconds (3) 48 seconds (4) 72 seconds (5) 96 seconds

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-li...
Questions

Physics, 18.12.2020 01:30


Mathematics, 18.12.2020 01:30


Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30



Arts, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30

Chemistry, 18.12.2020 01:30


English, 18.12.2020 01:30





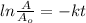
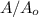 will become 0.125
will become 0.125