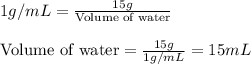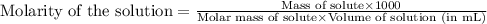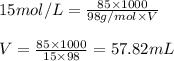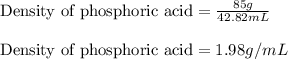Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Concentrated phosphoric acid is sold as a solution of 85% phosphoric acid and 15% water by mass. Giv...
Questions

Mathematics, 07.08.2021 17:10


Mathematics, 07.08.2021 17:10



Mathematics, 07.08.2021 17:10

Mathematics, 07.08.2021 17:10


Mathematics, 07.08.2021 17:10

Physics, 07.08.2021 17:10

Biology, 07.08.2021 17:10

English, 07.08.2021 17:10



Physics, 07.08.2021 17:20

Mathematics, 07.08.2021 17:20

English, 07.08.2021 17:20

Social Studies, 07.08.2021 17:20

Mathematics, 07.08.2021 17:20

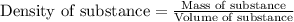 ......(1)
......(1)