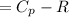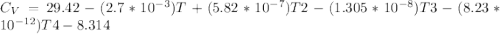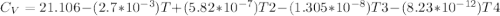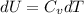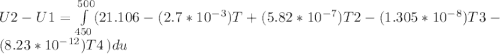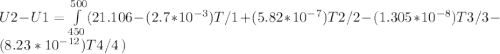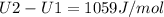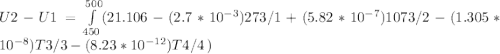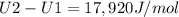The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 -...

Chemistry, 10.02.2020 23:04 steventhecool22
The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 - (2.170*10^-3 ) T + (0.0582*10^-5 ) T2 + (1.305*10^-8 ) T3 – (0.823*10^-11) T4
T in K and Cp in Joule/(mole-K). Assuming that N2 is an ideal gas:
A) How much internal energy (per mole) must be added to nitrogen to increase its temperature from 450 to 500 K. B) Repeat part A for an initial temperature of 273 K and final temperature of 1073 K.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2

Chemistry, 23.06.2019 15:40
Glucose (c6h12o6) is the simple sugar that plants make. what is the total number of atoms in glucose? 1 3 24 144
Answers: 1
You know the right answer?
Questions


Geography, 24.11.2020 22:10


History, 24.11.2020 22:10

Mathematics, 24.11.2020 22:10


Spanish, 24.11.2020 22:10

History, 24.11.2020 22:10

Advanced Placement (AP), 24.11.2020 22:10

Mathematics, 24.11.2020 22:10






Mathematics, 24.11.2020 22:10

Geography, 24.11.2020 22:10

Social Studies, 24.11.2020 22:10

Mathematics, 24.11.2020 22:10

History, 24.11.2020 22:10

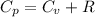
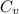 from above if we make
from above if we make