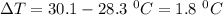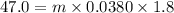Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
The specific heat of lead is 0.0380 cal/g·°C. If 47.0 calories of energy raised the temperature of a...
Questions

English, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10


Mathematics, 04.02.2021 22:10


English, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Arts, 04.02.2021 22:10



Mathematics, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

Social Studies, 04.02.2021 22:10




Mathematics, 04.02.2021 22:10


Mathematics, 04.02.2021 22:10

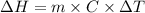
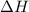 is the enthalpy change
is the enthalpy change
 is the temperature change
is the temperature change