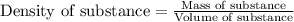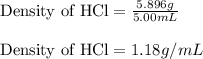Chemistry, 11.02.2020 00:09 ichabella2010
Experimental Procedure, Part C.3. The mass of a beaker is 5.333g. After 5.00mL of a concentrated hydrochloric acid solution is pipetted into the beaker, the combined mass of the beaker and the hydrochloric acid sample is 11.229g. From the data, what is the measured density of the hydrochloric acid solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Experimental Procedure, Part C.3. The mass of a beaker is 5.333g. After 5.00mL of a concentrated hyd...
Questions

Mathematics, 24.12.2019 20:31


Physics, 24.12.2019 20:31

Mathematics, 24.12.2019 20:31

History, 24.12.2019 20:31

Mathematics, 24.12.2019 20:31

Biology, 24.12.2019 20:31


Chemistry, 24.12.2019 20:31

Mathematics, 24.12.2019 20:31


Mathematics, 24.12.2019 20:31



Social Studies, 24.12.2019 20:31