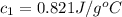Chemistry, 11.02.2020 01:14 treseanthegreat
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 20.0 °C. The final temperature of the system is 25.0 °C. Determine the specific heat of the metal.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 2...
Questions


Chemistry, 26.05.2021 19:50

Mathematics, 26.05.2021 19:50

English, 26.05.2021 19:50







Biology, 26.05.2021 19:50

Mathematics, 26.05.2021 19:50



Arts, 26.05.2021 19:50


Mathematics, 26.05.2021 19:50



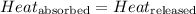
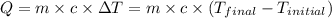
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0505/6952/09236.png) ......(1)
......(1)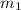 = mass of metal = 30 g
= mass of metal = 30 g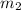 = mass of water = 100 g
= mass of water = 100 g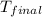 = final temperature = 25°C
= final temperature = 25°C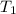 = initial temperature of metal = 110°C
= initial temperature of metal = 110°C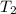 = initial temperature of water = 20.0°C
= initial temperature of water = 20.0°C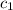 = specific heat of metal = ?
= specific heat of metal = ?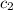 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![30\times c_1\times (25-110)=-[100\times 4.186\times (25-20)]](/tpl/images/0505/6952/ee389.png)