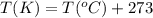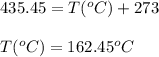Chemistry, 11.02.2020 02:21 Ladarius566
What is the boiling point of benzene if the external pressure is 5500 torr? Hvap Benzene = 30.72 kJ/mol Normal boiling point of 80.1oC R = 8.314J/ mol K

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
What is the boiling point of benzene if the external pressure is 5500 torr? Hvap Benzene = 30.72 kJ/...
Questions

Mathematics, 15.01.2021 23:20



Mathematics, 15.01.2021 23:20





Mathematics, 15.01.2021 23:20


Mathematics, 15.01.2021 23:20

Arts, 15.01.2021 23:20


Mathematics, 15.01.2021 23:20


Mathematics, 15.01.2021 23:20

Chemistry, 15.01.2021 23:20



Mathematics, 15.01.2021 23:20

![\ln(\frac{P_2}{P_1})=\frac{\Delta H_{vap}}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0505/8524/b9a49.png)
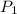 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr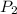 = final pressure which is external pressure = 5500 torr
= final pressure which is external pressure = 5500 torr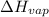 = Enthalpy of vaporization = 30.72 kJ/mol = 30720 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 30.72 kJ/mol = 30720 J/mol (Conversion factor: 1 kJ = 1000 J)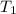 = initial temperature or normal boiling pont =
= initial temperature or normal boiling pont = ![80.1^oC=[80.1+273]K=353.1K](/tpl/images/0505/8524/07b85.png)
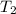 = final temperature = ?
= final temperature = ?![\ln(\frac{5500}{760})=\frac{30720J/mol}{8.314J/mol.K}[\frac{1}{353.1}-\frac{1}{T_2}]\\\\T_2=435.45K](/tpl/images/0505/8524/ff809.png)