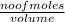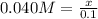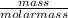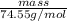Chemistry, 11.02.2020 02:27 lmcginnis2003
Suppose you were making 100.0 mL of a solution that is 0.040 M KCl. What mass of KCl should you weigh to make this solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

You know the right answer?
Suppose you were making 100.0 mL of a solution that is 0.040 M KCl. What mass of KCl should you weig...
Questions


History, 23.07.2019 16:30

Mathematics, 23.07.2019 16:30

Mathematics, 23.07.2019 16:30






History, 23.07.2019 16:30





English, 23.07.2019 16:30

Spanish, 23.07.2019 16:30


Business, 23.07.2019 16:30

Computers and Technology, 23.07.2019 16:30

History, 23.07.2019 16:30