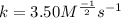Chemistry, 11.02.2020 03:01 carter4026
Consider the equation:
CHCl3(g)+Cl2(g) > CCl4(g)+HCl(g)
The initial rate of the reaction is measured at several different concentrations of the reactants with the following results: [CHCl3](M) [Cl2](M) Initial rate (M/s)
0.010 0.010 0.0035
0.020 0.010 0.0069
0.020 0.020 0.0098
0.040 0.040 0.027
1. From the data, choose the correct rate law for the reaction.
Rate = k[CHCl3][Cl2]^2Rate = k[CHCl3]^2[Cl2]^1/2Rate = k[CHCl3][Cl2]Rate = k[CHCl3][Cl2]^1/2
2. the rate constant (k) for the reaction. Express your answer using three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

You know the right answer?
Consider the equation:
CHCl3(g)+Cl2(g) > CCl4(g)+HCl(g)
The initial rate of the reac...
CHCl3(g)+Cl2(g) > CCl4(g)+HCl(g)
The initial rate of the reac...
Questions

Arts, 30.01.2021 05:00


Chemistry, 30.01.2021 05:00


Mathematics, 30.01.2021 05:00

Mathematics, 30.01.2021 05:00



English, 30.01.2021 05:00

History, 30.01.2021 05:00

Arts, 30.01.2021 05:00


Mathematics, 30.01.2021 05:00

Mathematics, 30.01.2021 05:00


Mathematics, 30.01.2021 05:00


Mathematics, 30.01.2021 05:00


Mathematics, 30.01.2021 05:00

![Rate=k[CHCl_3]^1[Cl_2]^\frac{1}{2}](/tpl/images/0505/9699/bea07.png)
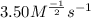
![rate=k[CHCl_3]^x[Cl_2]^y](/tpl/images/0505/9699/ba01c.png)
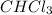
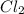
![0.0035=k[0.010]^x[0.010]^y](/tpl/images/0505/9699/b387a.png) (1)
(1)
![0.0069=k[0.020]^x[0.010]^y](/tpl/images/0505/9699/ae150.png) (2)
(2)
![\frac{0.0069}{0.035}=\frac{k[0.020]^x[0.010]^y}{k[0.010]^x[0.010]^y}](/tpl/images/0505/9699/79be9.png)
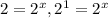 therefore x=1.
therefore x=1.![0.0098=k[0.020]^x[0.020]^y](/tpl/images/0505/9699/46f00.png) (4)
(4)
![\frac{0.0098}{0.0069}=\frac{k[0.020]^x[0.020]^y}{k[0.020]^x[0.010]^y}](/tpl/images/0505/9699/470b5.png)
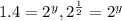 therefore
therefore 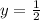
![rate=k[CHCl_3]^1[Cl_2]^\frac{1}{2}](/tpl/images/0505/9699/cc117.png)
![0.0035=k[0.010]^1[0.010]^\frac{1}{2}](/tpl/images/0505/9699/a917e.png)