Chemistry, 11.02.2020 02:58 derrishamckenzie22
Given the data calculated in Parts A, B, C, and D, determine the initial rate for a reaction that starts with 0.45 M of reagent A and 0.90 M of reagents B and C?
Trial [A]
(M) [B]
(M) [C]
(M) Initial rate
(M/s)
1 0.20 0.20 0.20 6.0×10−5
2 0.20 0.20 0.60 1.8×10−4
3 0.40 0.20 0.20 2.4×10−4
4 0.40 0.40 0.20 2.4×10−4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Given the data calculated in Parts A, B, C, and D, determine the initial rate for a reaction that st...
Questions


Mathematics, 02.07.2019 00:30




Mathematics, 02.07.2019 00:30




Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30


Mathematics, 02.07.2019 00:30

Physics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30

Computers and Technology, 02.07.2019 00:30

Health, 02.07.2019 00:30


Mathematics, 02.07.2019 00:30


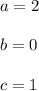
![rate=k[A]^a{B}^b[C]^c\\ \\ rate=k[A]^2[B]^0[C]^1=k[A]^2C](/tpl/images/0505/9538/57b95.png)
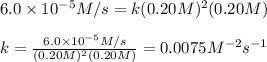
![rate=k[A]^2C=0.0075[A]^2[C]](/tpl/images/0505/9538/d0e9c.png)
![rate=0.0075M^{-2}s^{-1}[A]^2[C]=0.0075M^{-2}s^{-1}[0.45M]^2[0.9M]](/tpl/images/0505/9538/e3157.png)