
Chemistry, 11.02.2020 03:23 kookycookiefanx
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C) in the absence of a catalyst. Assuming that the frequency factor remains the same, by how much must an enzyme lower the activation energy of the reaction to achieve a million- fold (1.0 x 106) increase in the reaction rate constant?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C)...
Questions


Mathematics, 27.01.2022 15:40

Social Studies, 27.01.2022 15:40

Social Studies, 27.01.2022 15:40











Mathematics, 27.01.2022 15:50


Chemistry, 27.01.2022 15:50

Mathematics, 27.01.2022 15:50

English, 27.01.2022 15:50

History, 27.01.2022 15:50

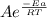
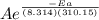
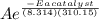
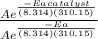 = 1.0 x 10⁶ by taking natural logarithm of both sides, we have
= 1.0 x 10⁶ by taking natural logarithm of both sides, we have


