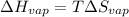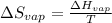Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
You know the right answer?
The boiling point of ethanol is 78.4 °C, and the enthalpy change for the conversion of liquid to vap...
Questions


Mathematics, 13.05.2021 18:00


History, 13.05.2021 18:00

Mathematics, 13.05.2021 18:00

Chemistry, 13.05.2021 18:00



Mathematics, 13.05.2021 18:00


Biology, 13.05.2021 18:00



Mathematics, 13.05.2021 18:00


Mathematics, 13.05.2021 18:00

Biology, 13.05.2021 18:00

Mathematics, 13.05.2021 18:00

English, 13.05.2021 18:00

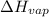 = 38.56 kJ/mol
= 38.56 kJ/mol