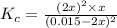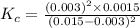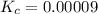Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
A 0.15 mol sample of H2S is placed in a 10 L reaction vessel and heated to 1132◦C. At equilibrium, 0...
Questions

English, 13.01.2021 20:50

History, 13.01.2021 20:50




English, 13.01.2021 20:50


Mathematics, 13.01.2021 20:50

English, 13.01.2021 20:50

Spanish, 13.01.2021 20:50

Mathematics, 13.01.2021 20:50

Mathematics, 13.01.2021 20:50



Social Studies, 13.01.2021 20:50





Mathematics, 13.01.2021 21:00

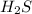 = 0.15 mole
= 0.15 mole
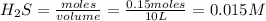
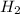 at equilibrium= 0.03 mole
at equilibrium= 0.03 mole
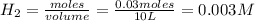 [/tex]
[/tex]
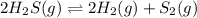
![K_c=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0506/3061/13e21.png)