
Chemistry, 11.02.2020 19:35 mohamedramadan
Equimolar solutions of copper(II) chloride, aluminum chloride, lithium chloride and calcium chloride are prepared. Which solution would be expected to have the highest conductivity? Hint: write the chemical formulas.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Equimolar solutions of copper(II) chloride, aluminum chloride, lithium chloride and calcium chloride...
Questions

English, 11.02.2021 17:00


Mathematics, 11.02.2021 17:00



History, 11.02.2021 17:00




Mathematics, 11.02.2021 17:10


History, 11.02.2021 17:10

Mathematics, 11.02.2021 17:10

Chemistry, 11.02.2021 17:10





Mathematics, 11.02.2021 17:10

Mathematics, 11.02.2021 17:10

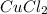
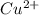 ions and 2 moles of
ions and 2 moles of 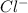 ions
ions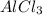
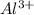 ions and 3 moles of
ions and 3 moles of 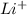 ions and 1 mole of
ions and 1 mole of 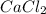
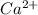 ions and 2 moles of
ions and 2 moles of 

