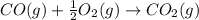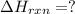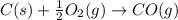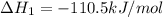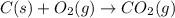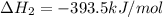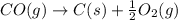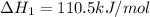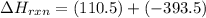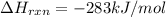CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a) Determine the value of the standard enthalpy change, ∆HrxnD , for the combustion of CO(g) at 298 Kusing the following information. C(s) + 12 O2(g) → CO(g) ∆H298D = − 110.5 kJ mol−1 C(s) + O2(g) → CO2(g) ∆H298D = − 393.5 kJ mol−1

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1
You know the right answer?
CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a)...
Questions





Mathematics, 12.10.2020 09:01

Mathematics, 12.10.2020 09:01




Mathematics, 12.10.2020 09:01



Physics, 12.10.2020 09:01

Social Studies, 12.10.2020 09:01




Social Studies, 12.10.2020 09:01


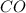 will be,
will be,