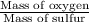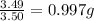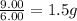Chemistry, 11.02.2020 20:15 davidsouth444
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these were decomposed the sulfur dioxide produced 3.49g oxygen and 3.50g sulfur, while the sulfur trioxide produced 9.00g oxygen and 6.00g sulfur. A) Calculate the mass of oxygen per gram of sulfur for sulfur dioxide. B) Calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 11:10
Why would a doctor most likely restrict a patient's contact with other people while the patient receives internal radiation
Answers: 1
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these were decompose...
Questions





Mathematics, 10.03.2020 06:44

History, 10.03.2020 06:45

Computers and Technology, 10.03.2020 06:45

Mathematics, 10.03.2020 06:45










History, 10.03.2020 06:46