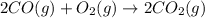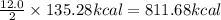Chemistry, 11.02.2020 20:55 7letters22
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g)∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide? Use only the information provided in this question. 1. 270.56 kcal 2. 1623.36 kcal 3. 811.68 kcal 4. 405.84 kcal 5. 541.12 kcal 6. 135.28 kcal

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
When molecules of water vapor in the air come in contact with a cold can of soft drink , they lose energy ,slow down,and form a liquid due to decrease in ?
Answers: 2


Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions








Biology, 11.11.2020 17:50

History, 11.11.2020 17:50





History, 11.11.2020 17:50




Mathematics, 11.11.2020 17:50