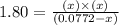Chemistry, 11.02.2020 21:05 lilpeepxliltracy
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) K c = 1.80 at 250 ∘ C A 0.166 mol sample of PCl 5 ( g ) is injected into an empty 2.15 L reaction vessel held at 250 ∘ C. Calculate the concentrations of PCl 5 ( g ) and PCl 3 ( g ) at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g...
Questions




Mathematics, 14.07.2020 23:01






English, 14.07.2020 23:01






Computers and Technology, 14.07.2020 23:01




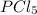 and
and 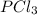 at equilibrium is, 0.0031 M and 0.0741 M respectively.
at equilibrium is, 0.0031 M and 0.0741 M respectively.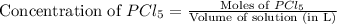
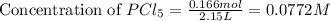
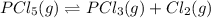
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0507/1118/73fe0.png)