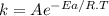The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln(k) on the y-axis and 1/T on the x-axis, and a best fit line with a slope of -10,473 K is obtained. Based on this data what is the activation energy of this reaction (in kJ/mol)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


You know the right answer?
The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln...
Questions


Mathematics, 03.12.2020 04:20

Advanced Placement (AP), 03.12.2020 04:20

English, 03.12.2020 04:20


Mathematics, 03.12.2020 04:20

Mathematics, 03.12.2020 04:20



Mathematics, 03.12.2020 04:20



Mathematics, 03.12.2020 04:20


Mathematics, 03.12.2020 04:20


Mathematics, 03.12.2020 04:20

Mathematics, 03.12.2020 04:20

Mathematics, 03.12.2020 04:20