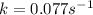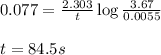Chemistry, 11.02.2020 23:54 AutumnGarringer
Suppose the half-life is 9.0 s for a first order reaction and the reactant concentration is 0.0741 M 50.7 s after the reaction starts. How many seconds after the start of the reaction does it take for the reactant concentration to decrease to 0.0055 M?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

You know the right answer?
Suppose the half-life is 9.0 s for a first order reaction and the reactant concentration is 0.0741 M...
Questions

Mathematics, 22.04.2021 01:50


Biology, 22.04.2021 01:50

Mathematics, 22.04.2021 01:50

Mathematics, 22.04.2021 01:50




Mathematics, 22.04.2021 01:50

Mathematics, 22.04.2021 01:50

Social Studies, 22.04.2021 01:50


Mathematics, 22.04.2021 01:50


Mathematics, 22.04.2021 01:50



English, 22.04.2021 01:50


Mathematics, 22.04.2021 01:50

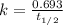
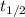 = half-life of the reaction = 9.0 s
= half-life of the reaction = 9.0 s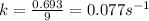
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0507/4595/f1041.png) ......(1)
......(1)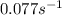
![[A_o]](/tpl/images/0507/4595/dc622.png) = initial amount of the reactant = ?
= initial amount of the reactant = ?![0.077=\frac{2.303}{50.7}\log\frac{[A_o]}{0.0741}](/tpl/images/0507/4595/3671b.png)
![[A_o]=3.67M](/tpl/images/0507/4595/2dfb2.png)
![[A]=0.0055M](/tpl/images/0507/4595/a1973.png)