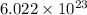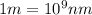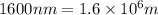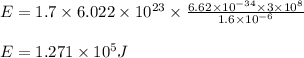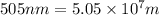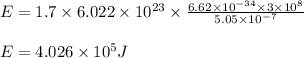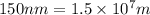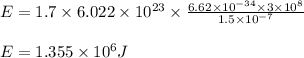Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

You know the right answer?
Determine the energy of 1.70 mol of photons for each of the following kinds of light. (Assume three...
Questions



History, 24.04.2021 06:30

Mathematics, 24.04.2021 06:30

Biology, 24.04.2021 06:30







Mathematics, 24.04.2021 06:30

Mathematics, 24.04.2021 06:30


Mathematics, 24.04.2021 06:30

English, 24.04.2021 06:30


Mathematics, 24.04.2021 06:30

Advanced Placement (AP), 24.04.2021 06:30

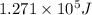

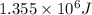
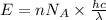 ......(1)
......(1)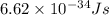
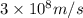
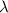 = wavelength of light
= wavelength of light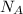 = Avogadro's number =
= Avogadro's number =