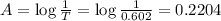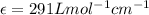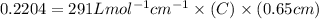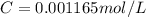Chemistry, 12.02.2020 02:47 batman48000
The molar extinction coefficient of cytochrome P450, one of the compounds involved in photosynthesis, at 522 nm is 291 L mol-1cm-1. When light of that wavelength passes through a cell of length 6.5 mm containing a solution of P450, 39.8 % of the light was absorbed. What is the molar concentration of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
The molar extinction coefficient of cytochrome P450, one of the compounds involved in photosynthesis...
Questions


Chemistry, 15.07.2019 16:20




Biology, 15.07.2019 16:20


History, 15.07.2019 16:20

Health, 15.07.2019 16:20

Mathematics, 15.07.2019 16:20




Mathematics, 15.07.2019 16:20






Mathematics, 15.07.2019 16:20


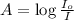
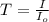
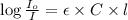
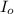 = incident light
= incident light = transmitted light
= transmitted light = molar absorptivity coefficient
= molar absorptivity coefficient