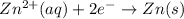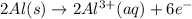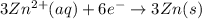The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented above. Based on the half-reactions, what is the coefficient for Al(s) if the equation for the oxidation-reduction reaction is balanced with the smallest whole-number coefficients?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2


Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented a...
Questions


Mathematics, 26.02.2020 05:55







Computers and Technology, 26.02.2020 05:55





Mathematics, 26.02.2020 05:55






Biology, 26.02.2020 05:55