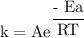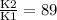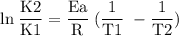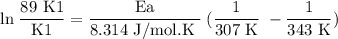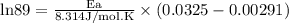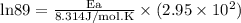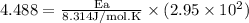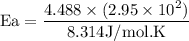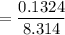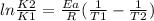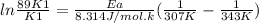Chemistry, 12.02.2020 03:19 CoolRahim9090
What is the activation energy (in kJ/mol) of a reaction whose rate constant increases by a factor of 89 upon increasing the temperature from 307 K to 343 K? R = 8.314 J/(mol • K). Only enter the numerical value as an integer in the answer box below. Do NOT type in the unit (kJ/mol).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
What is the activation energy (in kJ/mol) of a reaction whose rate constant increases by a factor of...
Questions


Mathematics, 05.03.2020 22:58

Mathematics, 05.03.2020 22:58

Mathematics, 05.03.2020 22:58

Geography, 05.03.2020 22:58

Mathematics, 05.03.2020 22:59






Mathematics, 05.03.2020 22:59

English, 05.03.2020 22:59